
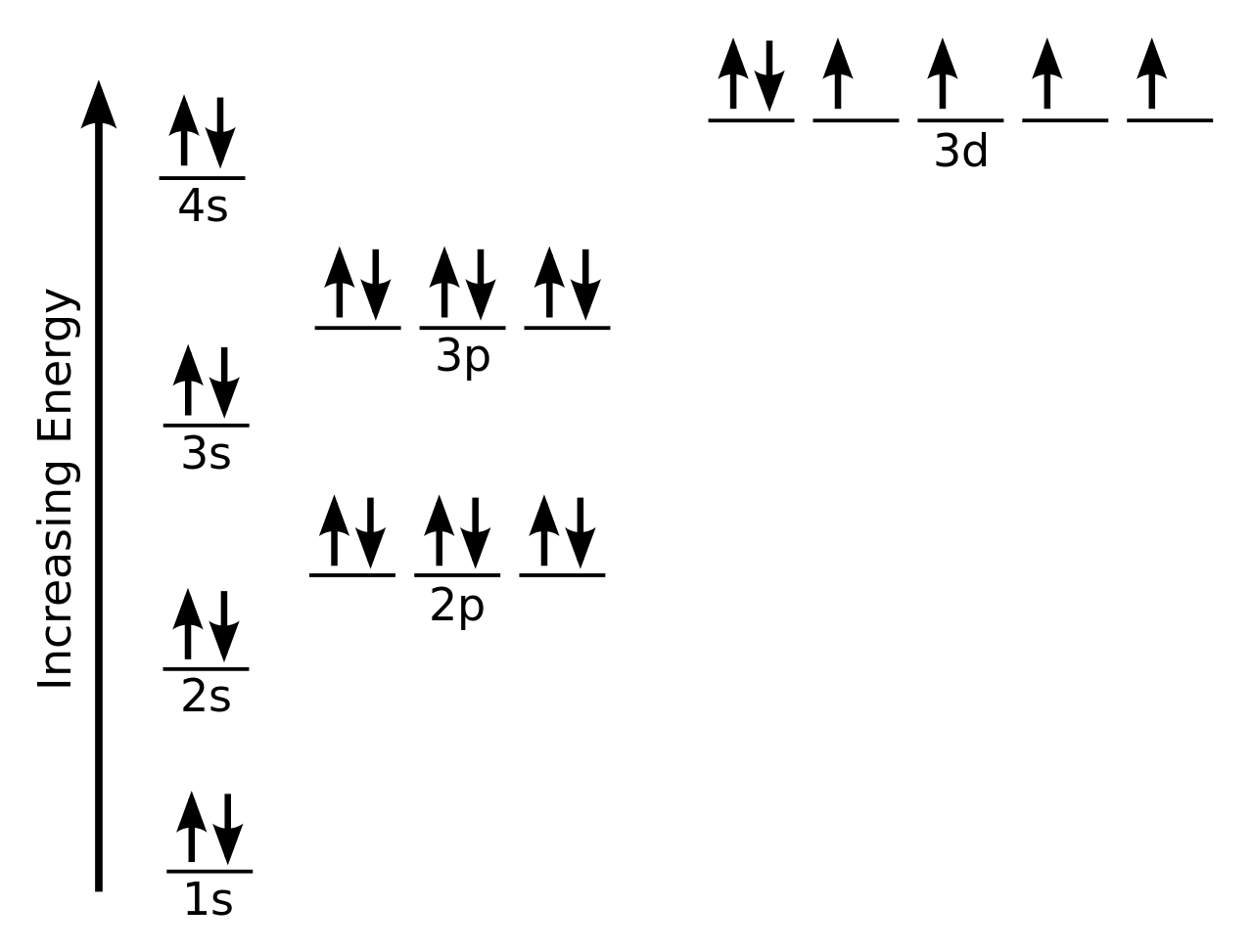
By “building up” from hydrogen, this table can be used to determine the electron configuration for any atom on the periodic table. This periodic table shows the electron configuration for each subshell. For example, after filling the 3 p block up to Ar, we see the orbital will be 4s (K, Ca), followed by the 3 d orbitals. The filling order simply begins at hydrogen and includes each subshell as you proceed in increasing Z order. Since the arrangement of the periodic table is based on the electron configurations, provides an alternative method for determining the electron configuration. illustrates the traditional way to remember the filling order for atomic orbitals. Electrons enter higher-energy subshells only after lower-energy subshells have been filled to capacity. Each added electron occupies the subshell of lowest energy available (in the order shown in ), subject to the limitations imposed by the allowed quantum numbers according to the Pauli exclusion principle.
This procedure is called the Aufbau principle, from the German word Aufbau (“to build up”). Beginning with hydrogen, and continuing across the periods of the periodic table, we add one proton at a time to the nucleus and one electron to the proper subshell until we have described the electron configurations of all the elements. To determine the electron configuration for any particular atom, we can “build” the structures in the order of atomic numbers. The notation 3 d 8 (read “three–d–eight”) indicates eight electrons in the d subshell (i.e., l = 2) of the principal shell for which n = 3. A superscript number that designates the number of electrons in that particular subshell.įor example, the notation 2 p 4 (read “two–p–four”) indicates four electrons in a p subshell ( l = 1) with a principal quantum number ( n) of 2.The letter that designates the orbital type (the subshell, l), and.The number of the principal quantum shell, n,.We describe an electron configuration with a symbol that contains three pieces of information ( ):

The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. We will discuss methods for remembering the observed order. For small orbitals (1 s through 3 p), the increase in energy due to n is more significant than the increase due to l however, for larger orbitals the two trends are comparable and cannot be simply predicted. Electrons in orbitals that experience more shielding are less stabilized and thus higher in energy. This phenomenon is called shielding and will be discussed in more detail in the next section. Electrons that are closer to the nucleus slightly repel electrons that are farther out, offsetting the more dominant electron–nucleus attractions slightly (recall that all electrons have −1 charges, but nuclei have + Z charges). Within each shell, as the value of l increases, the electrons are less penetrating (meaning there is less electron density found close to the nucleus), in the order s > p > d > f. But this is not the only effect we have to take into account. Thus, the attraction to the nucleus is weaker and the energy associated with the orbital is higher (less stabilized). As the principal quantum number, n, increases, the size of the orbital increases and the electrons spend more time farther from the nucleus. The filling order is based on observed experimental results, and has been confirmed by theoretical calculations. Thus, many students find it confusing that, for example, the 5 p orbitals fill immediately after the 4 d, and immediately before the 6 s. Generalized energy-level diagram for atomic orbitals in an atom with two or more electrons (not to scale).Įlectrons in successive atoms on the periodic table tend to fill low-energy orbitals first.


 0 kommentar(er)
0 kommentar(er)
