
DEFINE PRECIPITATE CHEMISTRY TERMS FREE
The more you know about how the reaction occurs, and the more you know about the properties of different solvents (like their polarity), the more educated of a guess you can make! For example, in double replacement reactions, we know that the solubility of the reactants is important because we need free ions around. In general, it's tricky to predict for any random reaction what medium it might need. Water is a really great solvent whenever you want to have ions around. Double replacement reactions always occur in water, with the reactants in the aqueous state.

another solid during a chemical reaction or by diffusion in a solid. Luckily, there aren't that many strong acids and bases, and you can learn morem about this from this video: Īnything that is soluble in water and dissolved (separated into individual cations and anions) is in the aqueous state. The definition of precipitation in Dictionary is as: Any or all of the forms of. Chemical reactions which are characterised by the formation of insoluble solid substances are called precipitates. Precipitates are insoluble in the solvent and can be. It is helpful to have the strong acids and bases memorized, since they have special reactivity. Definition: The simultaneous precipitation of a normally soluble component with a macro-component from the same solution by the formation of mixed crystals, by. A precipitate is a solid that forms from a solution during a chemical reaction. The cation (or positively charged ion) of the salt comes from the base, and the anion (or negatively charged ion) comes from the acid. Fehling's solution is blue due to dissolved Cu 2+. In meteorology a precipitate is liquid or solid water (rain, snow, etc.) falling from the sky. Examples: Table salt, NaCl, is an ionic compound.
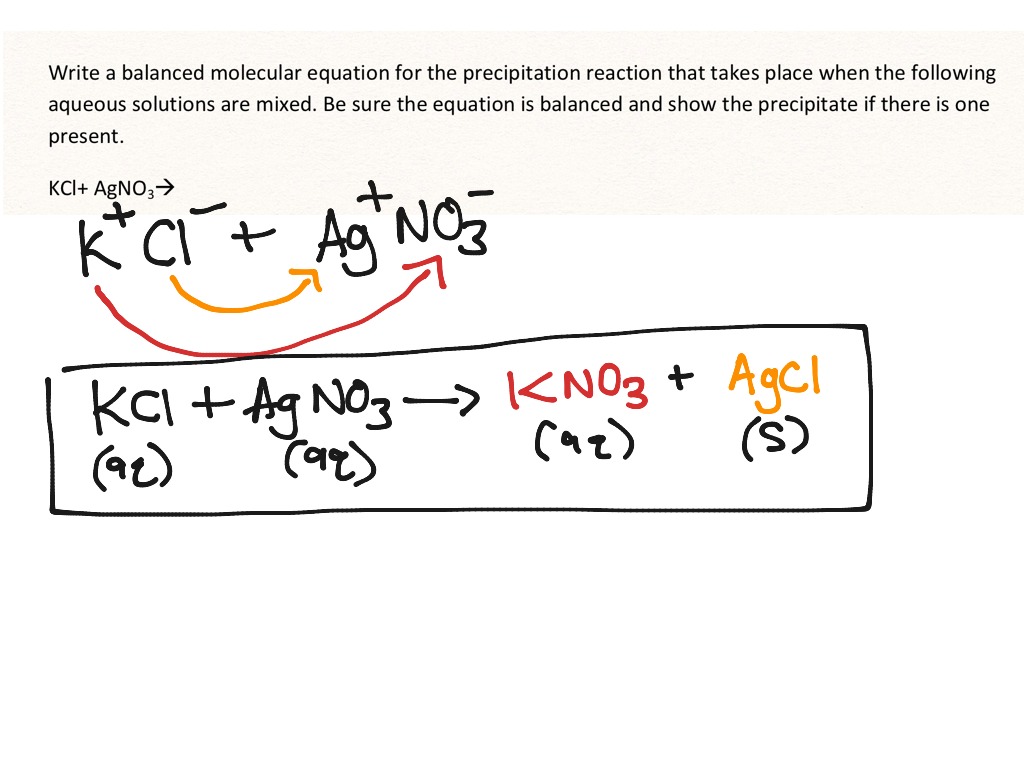
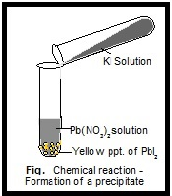
If you have tried this reaction at home, you probably remember a lot of fizzing because the neutralization reaction is accompanied by a gas-producing reaction, where the carbonic acid decomposes into carbon dioxide gas-bubbles!-and water.Ī salt is generally any ionic compound, though I have also seen it defined as an ionic compound that is formed when you react an acid and a base. Precipitate: In chemistry, a solid formed by a change in a solution, often due to a chemical reaction or change in temperature that decreases solubility of a solid. Ionic Compound Definition: An ionic compound is a compound formed by ions bonding together through electrostatic forces. Commun.A + B − + C + D − → A + D − + C + B − \greenD NaCH 3 COO start text, N, a, C, H, end text, start subscript, 3, end subscript, start text, C, O, O, end text. Shengju Wu, Fengting Li, Yinan Wu, Ran Xu and Guangtao Li, Chem. Preparation of novel poly(vinyl alcohol)/SiO 2 composite nanofiber membranes with mesostructure and their application for removal of Cu 2+ from waste water Commun., 2009ĭendronized iron oxide nanoparticles as contrast agents for MRIīrice Basly, Delphine Felder-Flesch, Pascal Perriat, Claire Billotey, Jacqueline Taleb, Geneviève Pourroy and Sylvie Begin-Colin, Chem. Theivanayagam Muraliganth, Arumugam Vadivel Murugan and Arumugam Manthiram, Chem. Commun., 2009įacile synthesis of carbon-decorated single-crystalline Fe 3O 4 nanowires and their application as high performance anode in lithium ion batteries Hydrophilic multi-walled carbon nanotubes decorated with magnetite nanoparticles as lymphatic targeted drug delivery vehiclesĭong Yang, Feng Yang, Jianhua Hu, Jiang Long, Changchun Wang, Deliang Fu and Quanxing Ni, Chem. BioSyst., 2009Įlucidating the genesis of Bi 2MoO 6 catalyst by combination of synchrotron radiation experiments and Raman scatteringĬhanapa Kongmark, Vladimir Martis, Annick Rubbens, Caroline Pirovano, Axel Löfberg, Gopinathan Sankar, Elisabeth Bordes-Richard, Rose-Noëlle Vannier and Wouter Van Beek, Chem. When the reaction occurs, the solid formed is called the precipitate, and the liquid remaining above the solid is called the supernate. Phosphoproteomics-finally fulfilling the promise? Precipitation is the formation of a solid in a solution during a chemical reaction. Sankaran Murugesan and Vaidyanathan (Ravi) Subramanian, Chem.

Robust synthesis of bismuth titanate pyrochlore nanorods and their photocatalytic applications More about the RSC Chemical Methods Ontology (CMO)įabrication of living cellosomes of rod-like and rhombohedral morphologies based on magnetically responsive templates


 0 kommentar(er)
0 kommentar(er)
